This website provides information about the business field of CO.DON GmbH, which has taken over the business operations from CO.DON AG.
Information on CO.DON Aktiengesellschaft can be found at www.codon-aktiengesellschaft.de.
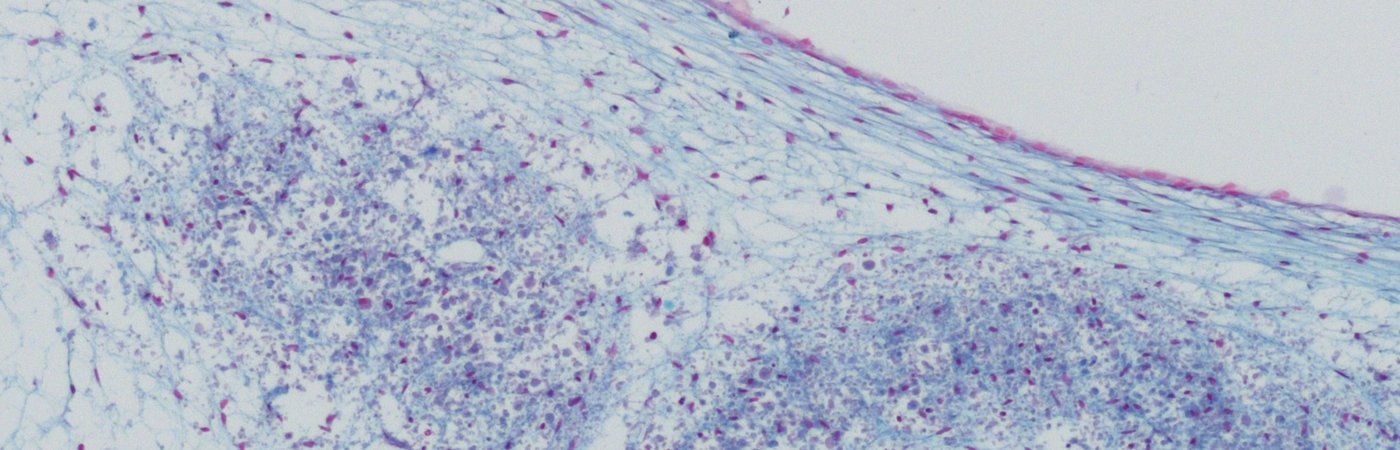
CO.DON has developed a procedure that enables the regenerative treatment of cartilage defects with autologous chondrocytes. The cartilage cell therapies consist of three-dimensional cell implants that are applied into the defect, where they naturally adhere. After implantation, they have the potential to synthesise hyaline-like cartilage. Autologous chondrocytes and the patient's own serum are used for manufacturing the cartilage cell implants. The method we have developed has been used successfully for more than 30 years. The safety and efficacy of our cartilage cell therapies has been confirmed in clinical studies1.
Treatment with our cell therapies is carried out using the regenerative method of matrix-associated autologous chondrocyte implantation (ACI-M). ACI with CO.DON’s method is the fourth generation of ACI2. It is a two-stage procedure that can be performed minimally invasive.
1 5-year follow-up data phase II (Hoburg A, Niemeyer P, Laute V, et al. OJSM. 2022;10(1). doi:10.1177/23259671211053380) and Phase III (Hoburg A, Niemeyer P, Laute V, et al. KSSTA. 2022 Oct 21. doi: 10.1007/s00167-022-07194-x).
2 QKG E.V. „Matrixinduzierte autologe Chondrozyten-Transplantation (M-ACT)“, QKG website, https://www.qkg-ev.de/fachinformationen/fuer-aerzte/mact/