This website provides information about the business field of CO.DON GmbH, which has taken over the business operations from CO.DON AG.
Information on CO.DON Aktiengesellschaft can be found at www.codon-aktiengesellschaft.de.

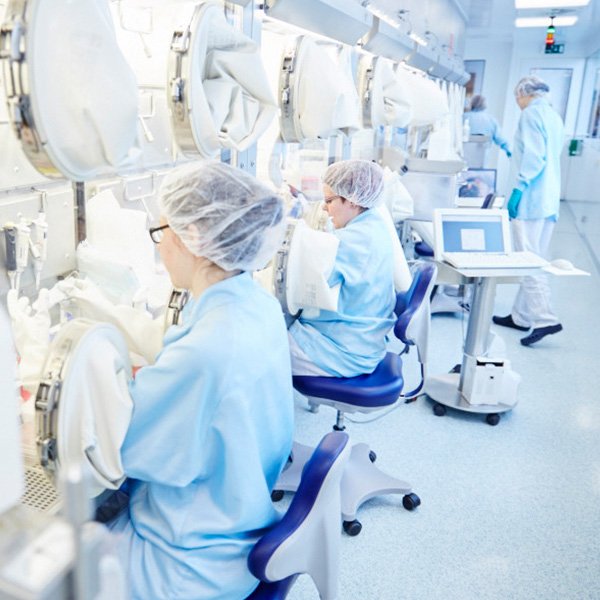
The Integrated Isolator Technology (IIT), our production facility, fulfils all the requirements of Good Manufacturing Practice (GMP), the applicable regulations for quality assurance of production processes and environments in the production of pharmaceutical products.
The special feature of the IIT is the "clean room within a clean room" principle, i.e. the integration of all the equipment required for implant production in isolators that meet the highest clean room standards. These isolators are located in an environment that is also classified as a clean room and are only accessible via airlock systems. Pressure differences between the different rooms prevent contamination between one room and another.
It was the demanding quality standards required of the manufacturing conditions for human cell implants that led us to develop Integrated Isolator Technology (IIT) alongside our industrial partners. In contrast to other pharmaceutical products, cell therapies or cell transplants cannot be sterilised due to their sensitivity to heat. A special production facility is therefore required to manufacture such products.
Our cell therapies are produced aseptically using only sterile materials. During the entire manufacturing process, the cells are monitored and regularly checked for contamination. At the end, each individual implant undergoes a strict approval process to ensure quality.
Our cell therapies are manufactured at the Teltow site. This is where the heart of CO.DON, the Integrated Isolator Technology (IIT), and the laboratories are located.
In the isolators, the cartilage cells are isolated, supplied and finally the cell implants are manufactured in the form of so-called spheroids. The isolator has all the equipment required for cell cultivation. This includes, for example, the incubators, a centrifuge, which is used for washing and moulding the spheroids and for separating cells and patient serum, a freezing unit and refrigerator, which are used to store certain materials for the production and testing of the cell transplants, as well as microscopes for regular cell quality checks.

In order for the cartilage cells to grow, it is necessary to simulate the environment in the human body. This is achieved with the help of incubators, which are located in the isolators and in which the necessary biochemical and physical properties for successful cell growth are maintained. These properties include sterility, a certain temperature, humidity and corresponding amount of carbon dioxide. The cells grow and multiply in cell culture flasks.
Material can be brought into the isolator via an airlock without affecting the sterile conditions in the isolator. To ensure the sterility of the materials and processes, the packaged starting materials for implant production, the required materials and the system itself are decontaminated with hydrogen peroxide (H2O2).


The cells can be supplied from the outside via glove ports in the isolators. This ensures that the cartilage cells can grow in a protected and germ-free environment, hermetically shielded. The gloves also ensure that our employees are protected. The gloves are arranged in such a way that employees can reach every point in the isolator.