This website provides information about the business field of CO.DON GmbH, which has taken over the business operations from CO.DON AG.
Information on CO.DON Aktiengesellschaft can be found at www.codon-aktiengesellschaft.de.

Cartilage is the shock absorber in our joints. Joint pain may be due to a cartilage defect – an injury or damage to the cartilage. Accidents or overexertion may be the cause, but the defects can also result from natural wear and tear. Cartilage cannot regenerate on its own. If the cartilage defect remains untreated, it could enlarge over time and the joint may deteriorate. However, there are regenerative treatment options available that make it possible to restore the structure and function of the defective cartilage.
The body's own cartilage cell implantation (matrix-associated autologous chondrocyte transplantation, ACI) can be an option for the regenerative treatment of cartilage defects. ACI is a two-stage surgical procedure that can be performed minimally invasive. It aims to regenerate damaged cartilage tissue and preserve the joint. In many cases, ACI can help to delay or prevent the use of an artificial joint.
Within the method of ACI, CO.DON has developed personalised cell therapies that consists of three-dimensional cartilage cell implants to be implanted into the defect with the aim to repair damaged cartilage. Our cell therapies can be used to treat cartilage defects in the knee, shoulder, ankle, hip and elbow.
CO.DON’s special technology allows for the manufacture of cell implants using nothing foreign to the patient’s body, only the patient’s own cartilage cells and blood serum. The implants are manufactured individually for each patient.
ACI with the CO.DON products has been used successfully for more than 30 years. Over 20,000 patients have already been treated. The safety and efficacy of our cartilage cell therapy has been confirmed in clinical studies1. Autologous cartilage cell implantation with the CO.DON products is considered the fourth generation of ACI2.
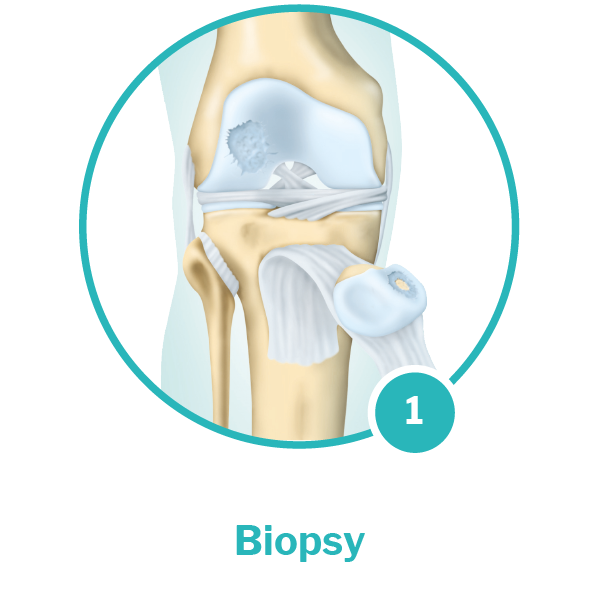

Biopsy: In a minimally invasive procedure*, a small sample of healthy cartilage tissue is removed from the affected joint. It is then sent to CO.DON, along with a blood sample from the patient.
Cultivation: At CO.DON‘s manufacturing facility, the cartilage cells are isolated and cultivated from the biopsy material using the blood sample. As they grow, they form small globules, the three-dimensional cartilage cell implants.
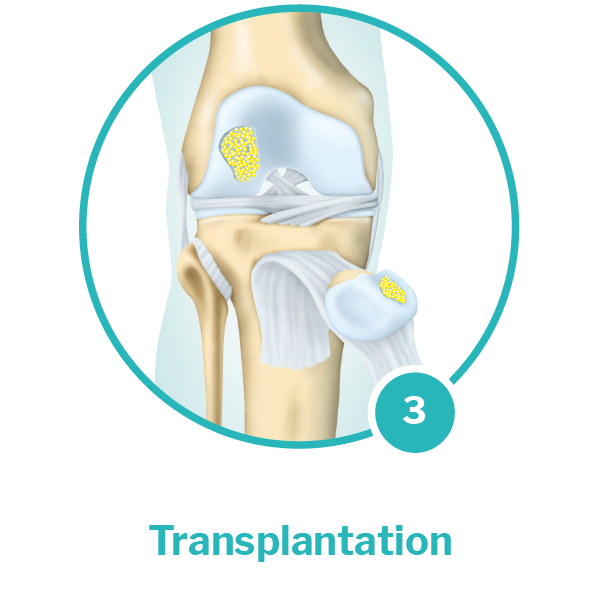
Implantation: In a second minimally invasive* procedure, the cartilage cell implants are implanted into the defect, to which they naturally adhere. Once applied into the defect, the tissue will naturally and gradually build up over the following weeks and months and integrate with the existing, healthy cartilage.
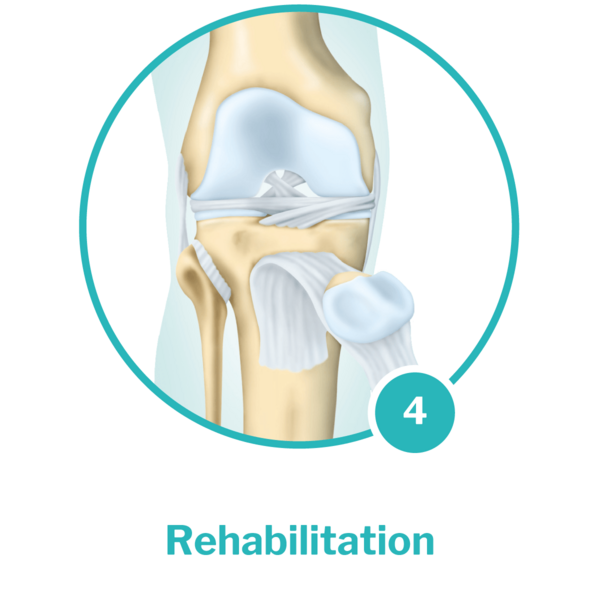
Rehabilitation: Once the cartilage cells have been implanted, a personalised rehabilitation plan begins. Once the therapy has been completed, the newly formed tissue is comparable with healthy cartilage – the joint can bear weight again.
*Recommended procedure
Professional ice hockey player Christopher Fischer suffered a knee injury with cartilage damage and reports on his successful return to sports after treatment for his cartilage defect. Take a look at his story.

1 5-year follow-up data phase II (Hoburg A, Niemeyer P, Lute V, et al. OJSM. 2022;10(1). doi:10.1177/23259671211053380) and Phase III (Hoburg A, Niemeyer P, Laute V, et al. KSSTA. 2022 Oct 21. doi: 10.1007/s00167-022-07194-x).
2 QKG E.V. „Matrixinduzierte autologe Chondrozyten-Transplantation (M-ACT)“, Website des QKG, https://www.qkg-ev.de/fachinformationen/fuer-aerzte/mact/.